News recently broke that the Russian government had approved a COVID-19 vaccine, appearing to give it bragging rights over the USA and other countries developing vaccines. They’ve even announced that members of President Putin’s family have been inoculated with the new vaccine. However, this Sputnik V vaccine is, like its namesake space probe, more a symbolic boast than anything. And unlike in the space race in 1958, Russia is actually behind in the COVID-19 vaccine race of 2020.
What drug approval really means
Drug approval operates country by country, and any government can “approve” anything they want. Moreover, exactly what constitutes “approval” differs from country to country. Some countries have multiple stages of approval that can’t really be compared to the approval processes in other countries. So, the Russian government has “approved” Sputnik V. But that doesn’t mean Sputnik V is the answer to our hopes — in terms of either medical evidence or production capacity.
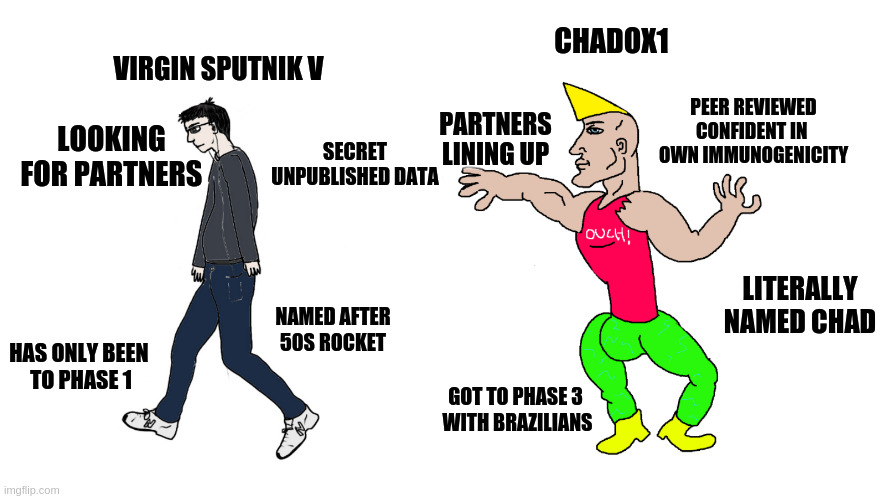
The Sputnik V vaccine is an adenovirus vector vaccine, most similar to the ChAdOx1 vaccine invented by Oxford University and produced by AstraZeneca and its international manufacturers. But compared to ChAdOx1, Sputnik V is actually very far behind:
- It has only passed small Phase 1 and Phase 2 trials with a non-final dosing schedule in dozens of patients, whereas ChAdOx1 has passed larger trials with a final dosing schedule in thousands of patients.
- The Phase 3 trial on Sputnik V has only just been announced and will only begin dosing in September, while ChAdOx1 has already enrolled over ten thousand patients over the last month.
- The data on Sputnik V is still being kept largely secret, while ChAdOx1 has been the subject of both clinical and preclinical peer-reviewed publications.
- AstraZeneca has spent the summer making production, packaging, and distribution agreements with a range of partners including Catalent, CEPI, GAVI, and the Serum Institute of India, while Sputnik V has only begun this process and has no firm production partners outside Russia.
- AstraZeneca and its partners are already setting up production facilities to produce up to 800 million doses in 2020, and two billion in 2021. Russia hopes to be producing 5 million doses of Sputnik V per month by the end of 2020, making it unlikely the country can achieve even domestic herd immunity using this vaccine in 2021, even if it does everything it says it’ll do.
This situation can be summarized thusly:
Some of the other vaccines, like the ones from Moderna and Pfizer/BiONTech, are similarly advanced to ChAdOx1. They have peer-reviewed evidence of immunogenicity, are already well into a Phase 3 trial, and set to produce a billion doses or more in 2021. Others, like those from Sinovac, Sinopharm, and CanSinoBio, all based in China, are behind these, but ahead of Sputnik V. Still others, like the protein vaccines from Novavax, Eli Lilly, etc, haven’t yet entered human trials, but have a much smoother path to producing billions of doses than Sputnik V does.
The bottom line: Sputnik V is several months behind the actual leaders, has less prospect of true mass production and gives no sign of catching up. Many of the manufacturing partners which could help Russia make more doses of Sputnik V are already executing agreements with competing vaccine programs that they made months ago.
Sputnik V approval is a gamble
The Russian government’s decision to “approve” Sputnik V is a boast, a gamble, an attempt to gain more support from other countries for its program, but it’s not the kind of considered judgment of safety and efficacy on the basis of firm clinical evidence which usually underlies a drug or vaccine approval in the developed world. They hope that by announcing this approval they can find more partners to manufacture it, and more countries to buy it than they would by being more reserved. And they can put great articles about it in their state press, including, of course, Sputnik International.
If it later turns out that Sputnik V works, Russia will have beaten the USA and other countries to the punch (nominally, even if not substantively). If not, well that’s why it’s a gamble. And while some in the press have used the phrase “Russian Roulette” to refer to this gamble, it’s actually relatively low stakes: this approval does not mean that Sputnik V will be widely distributed immediately. This approval doesn’t allow that (there’s a later stage approval that does), and a majority of Russian doctors surveyed don’t have confidence that it’s safe and effective. Even if they did think the vaccine would be effective, Russia won’t have large enough quantities to vaccinate people for months, at least.
It will be great if Sputnik V does work, and Russia can find partners to mass manufacture it (their announced target is one billion doses before the end of the pandemic). If current announcements from other firms all come true, the world will still not have enough doses for global herd immunity before the end of 2021. Any additional dose is a good thing, but right now, Russia’s vaccine doesn’t look likely to help the rest of the world. Who wouldn’t rather manufacture another vaccine that is further along?
We saw this same situation in miniature in China in July, when Cansino’s vaccine was cleared by the state research institute to enter a Phase 3 trial with PLA soldiers for subjects. Some media misreported this news as a final approval, but China was quick to dispel it. The Russian government, by contrast, in a longstanding tradition, seems happy to boast.
This news doesn’t really change anything. Sputnik V will still succeed if it gives good evidence of safety and efficacy in well-run trials and is mass manufactured. If not, it’ll fail. An experimental vaccine by another name is no more sweet.


You are reporting the comment """ by on